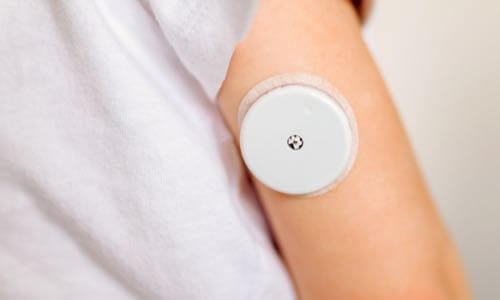
Continuous glucose monitoring (CGM) includes a sensor that is inserted subcutaneously under the skin, a transmitter, and a receiver.1 Most devices permit real-time glucose display to allow the individual to respond to changes in glucose values; all can
generate reports for later download and review. CGM is recognized as an important tool to enhance diabetes management and is recommended by the American Diabetes Association’s Standards of Medical Care for adults and children with diabetes.2
Evidence of benefits continues to grow for both Type 1 and Type 2 diabetes. 3,4
There are two types of CGM: personal CGM for home use and professional CGM, which is specifically designed for use by health care professionals and worn by the person with diabetes (PWD) on a short-term basis. It is imperative that the diabetes care and education specialist understand the advantages of CGM as compared to blood glucose monitoring (BGM) and hemoglobin A1C and maintain a high level of expertise in this area to best support the growing number of PWD that use these devices. The identify, configure, collaborate model and DATAA tool can help the diabetes care and education specialist to integrate CGM into their practice and optimize care for PWD.
Benefits of Personal CGM
Data from research studies have demonstrated that use of CGM leads to clinically significant reductions in hypoglycemia compared to a BGM alone in individuals with Type 1 diabetes.5-6
In a multicenter, randomized control trial (RCT) of 239 individuals, people with Type 1 diabetes on multiple daily injections, who utilized CGM, experienced a 38% overall reduction in hypoglycemia and a 40% reduction of nighttime duration of BG <
70 mg/dL.7
In an additional RCT of 224 individuals with Type 2 diabetes, the 149 individuals assigned to the intervention arm of the study experienced a 43% reduction in BG <70mg/dl with a 54% reduction in nocturnal hypoglycemia.8 Additional randomized
controlled trials show significant A1C reductions in adults9-10, older adults11, and children12. Use of CGM drives A1C reduction regardless of the type of insulin delivery system.13 Empowerment and quality
of life have also demonstrated improvements with CGM use.14
Professional CGM in Treatment
Continuous glucose monitors measure interstitial sensor glucose (SG), providing valuable information that is unattainable using finger stick capillary BGM. Professional Continuous Glucose Monitors can be used by health care professionals to guide
treatment decisions due to the availability of a large amount of data for pattern identification and management. The professional CGM devices are worn to collect information that will be downloaded and reviewed by a health care professional, providing
retrospective data.
There are currently two approved professional CGM devices in the U.S. The data cannot be integrated with insulin pumps or smart pens. The sensor glucose (SG) readings are recorded every one-to-fifteen minutes depending on device.
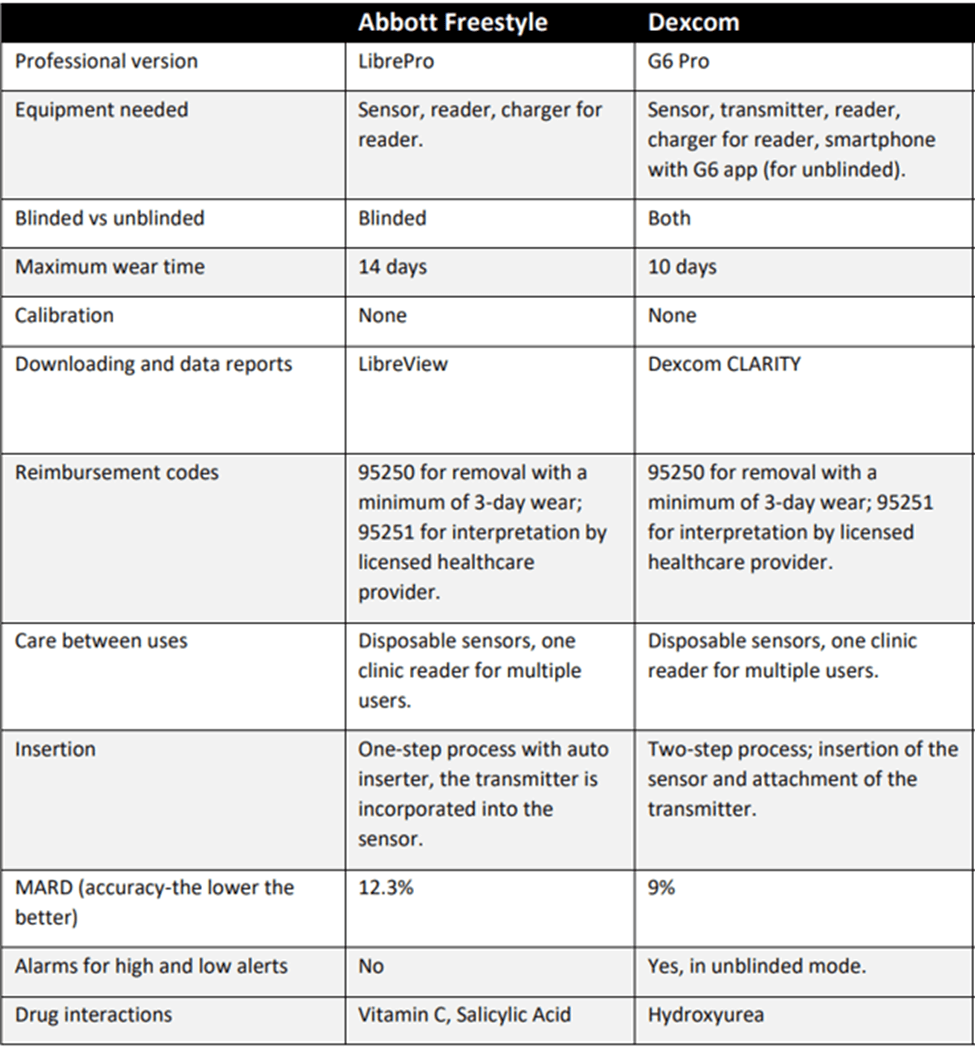
There are some differences between devices which are important for PWD and health care professionals to understand (Table 1). The following paragraphs describe the features the diabetes care and education specialist should be aware of when considering
professional CGM use.
a. Blinded versus Unblinded
Blinded CGM means that the PWD cannot see the SG readings in real time while wearing the device. This is intentional so that the PWD 1) does not alter their behavior based on the numbers they are seeing and 2) can wear the device for a short period of
time to gather retrospective glucose data on downloads to enhance treatment decision making by or with their health care provider.
Unblinded means the PWD can see their glucose readings in real time. There are advantages to each approach. In the blinded option, individuals are not aware of their glucose readings and may be more likely to go about their normal routine and activities
rather than making corrections to hyperglycemia and hypoglycemia when the data is visible. This may provide more realistic data for the health care professional to make medication and treatment adjustments. All three professional CGM devices are available
in blinded mode.
The Dexcom G6 Pro is the only professional CGM device that can be viewed in real time by the PWD and is also approved to make treatment decisions in real time. An advantage to unblinded, real-time visual CGM readings is that PWD can see and react to a
low glucose before it becomes severe and potentially prevent hypoglycemia through use of alerts if desired. The individual can also treat hyperglycemia sooner with the addition of high alarms and/or visual cues. Using unblinded CGM can also allow
the user to see in real time the effects of food, medication, activity and stress without waiting for the report to be downloaded.
Wearing and discussing the results of professional CGM is a great way to introduce the individual to personal CGM to
consider purchasing one for themselves. The Dexcom G6 Pro requires that a person have a compatible smart device and download the G6 mobile app to use in unblinded mode.
b. Cost
Cost can be a consideration when determining which device to purchase for the clinic.
The two available professional CGM devices have a one-time use sensor and transmitter and re-usable reader. There is a minimal expense if the sensor is lost or not returned. Sites can order a supply of the sensors so that they always have some
in stock, but they should be wary of expiration dates. When the sensor is returned, the stored information should be downloaded and reviewed with the PWD to note SG trends over the duration of wear and discuss management strategies.
c. Wear Time and Accuracy
The wear time is slightly different among devices and ranges from 10 to 14 days. The accuracy, reflected by the mean absolute relative difference (MARD),15 is also slightly different between devices. Some practices have
the PWD mail back the transmitter. Once received, the sensor can be downloaded and the information shared. When using the Dexcom G6 Pro in unblinded mode, the PWD could download the Clarity app and data can be viewed remotely. An appointment
should be made to review the data with the PWD, in person, by phone or virtually. See Section G on reimbursement. Of note, the Dexcom G6 Pro must be downloaded within 30 days of starting the session to retrieve data.
d. Sensor Insertion and Training
Diabetes care and education specialists must be trained on the proper insertion technique as well as cleaning and disinfecting the equipment between PWD if applicable. Product manufacturers may have a certification program to document that health care
professionals have completed the training on the device. Diabetes care and education specialists also have an important role in counseling PWD about certain restrictions during use of the device. Examples include: avoidance of CTs, MRIs,
diathermy and x-rays for all CGM devices. Certain medications can interfere with readings in some CGMs. See Interfering Substances and Procedures for CGM.
The diabetes care and education specialist should refer to the user guide of the device for potential contraindications. The diabetes care
and education specialist is poised to provide the additional teaching needed for systems such as setting alerts for high and low values as agreed upon with the individual. They can also review how to document events such as insulin doses,
grams of carbohydrates, exercise and health-related issues such as illness and infection, which may influence glucose values. Documenting events into the mobile app may be too complex for some individuals and can be handwritten instead. This
information is very beneficial when interpreting the downloaded data.
e. The Importance of Keeping a Food/Activity Log
Professional CGM is useful for teaching PWD about the effects of food, medications, physical activity and stress on their BG levels. Individuals are encouraged to track their food intake, physical activity and medication-taking behaviors which
provide much richer data when interpreting the CGM report. According to the AACE/ACE position statement on glucose monitoring,16 glucose pattern analysis may be used as an educational tool to demonstrate the relationship between
an individual’s glucose levels, their medication and other therapeutic interventions.
The two professional CGM companies have food and activity logs available, which can be obtained from company representatives, online or a general
log could also be created and distributed. There are many mobile apps also available, some of which allow the person to take pictures of their food.17
f. Individualizing Device Selection
All of the sensors and transmitters are waterproof and can be worn in the shower and bathtub regularly. It is important to note that while the sensors and transmitters can be worn in the pool as well, data may not transmit during this time because
of signal interference created by being submerged underwater. Diabetes care and education specialists can help health care providers identify which CGM device would be best for each individual. PWD may experience many challenges including visual
impairment, dexterity issues or other barriers to monitoring blood glucose. In these cases, a product that is easily inserted does not require calibration and does not demand frequent interaction may be the best choice.
g. Downloading CGM Data
Each device has a different software system into which the data is downloaded in clinic. There are slight differences in reports depending on the system used for download. All have the capability to show the CGM key metrics including time in range,
time spent below range and time spent above range. There is also glucose variability, glucose management indication (GMI) and the number of days worn. A graph superimposing all days of use also called an ambulatory glucose profile (AGP) helps
to determine trends and patterns. The complete AGP reports also includes a day-by-day breakdown.18
h. Professional CGM Billing/Reimbursement
For the purpose of data analysis and insurance reimbursement, professional CGM devices must be worn for at least 72 hours. Many insurance plans will cover professional CGM, but the process varies among plans with some requiring prior authorization.
It is recommended to verify insurance coverage prior to use. The device is inserted on the first visit which involves the use of a simple insertion process.
In 3 to 14 days, the PWD can either drop off the device for download or return for
a follow up visit and review the results with the diabetes care and education specialist or their diabetes health care provider. After documentation of a minimum of 72 hours of data, the provider can bill using the code 95250. Per reimbursement
guidelines, interpretation can be done remotely, so results can be mailed, discussed by phone, sent via Electronic Medical Records (EMR) or discussed through a virtual visit. Per Medicare guidelines, the final interpretation must be done by
a nurse practitioner, PA or physician, and is billed using a CPT code of 95251. Depending on state laws, pharmacists may also be able to perform the interpretation through a collaborative practice agreement. Diabetes care and education specialists
can review the download and make recommendations to the health care provider, who can then review and bill for the evaluation.
To view available professional and personal CGM, visit our Find & Compare CGMs page.
Personal CGM Specifics
a. How it Works
The personal CGM device collects data every 1-5 minutes and records data every 5–15 minutes depending on the device. The device displays SG and rate and direction of change, and may have the option to alarm the user about low and high SG
levels. This real-time data can help inform treatment decisions, give advanced warning of rapid glucose changes and motivate PWD to enhance their diabetes self-management.19 There are currently four companies that produce personal
CGM devices available in the U.S.
b. Considerations/Limitations
CGM minimizes the need for BG monitoring. However, finger stick BG checks are warranted in the following situations:
▪ A calibration or blood glucose symbol appears on the device.
▪ Symptoms or expectations do not match CGM readings.
▪ CGM readings are suspected to be inaccurate or used for an off-label indication like pregnancy (CGM use in pregnancy is approved for the Libre3 and Dexcom G7).
▪ Determining an insulin dose for a correction if the device is only FDA approved for adjunctive therapy.
However, there is the risk of increased distress in some individuals especially with devices that cause multiple alarms.20
Not all systems are approved for pregnant women, persons on dialysis or critically ill populations. Although currently not a standard of care, recent research studies support the utilization of CGM in these populations.21-25
c. Diabetes Care & Education Specialist’s Role
Diabetes care and education specialists well versed in CGM are poised to help in the selection of appropriate candidates, educate regarding device options, and instruct on sensor utilization. While evaluation of results is billable only by
a physician, nurse practitioner or PA, the diabetes care and education specialist may assist with downloading the devices and interpretation of the results. Providing information, skills and support will result in empowered individuals
who can embrace this innovative technology.
Comprehensive engagement includes management of CGM technology, evaluation of the results, and assessment of the needs and goals of the individual.26
Key teach-back points should include:
▪ Sensor site selection
▪ Insertion of the sensor
▪ Attachment (and charging) of the transmitter to the sensor, if required
▪ Required taping/securing of the sensor/transmitter
▪ Connection of the transmitter to the receiver
▪ Difference between SG and BG
▪ Understanding CGM data and trends
▪ Calibration including timing, frequency and importance of accurate meter/finger stick technique if required
Other topics that should be addressed are:
▪ Setting and managing alerts including high alert, low alert, high snooze, low snooze, rise rate, fall rate and predictive alerts
▪ Problem solving for site adhesiveness
▪ Support with coping and problem solving related to individual behavioral issues that can improve management
▪ Possible interference of products that include acetaminophen, hydroxyurea, tetracycline, salicylic acid and high-dose vitamin C. See Interfering Substances and Procedures for CGM.
▪ Education to prevent overcorrection of high glucose (some people will correct every five minutes or every 30 minutes when they see the numbers are not going down as quickly as they would like, which could lead to hypoglycemia)
▪ Sharing data: how and when to involve others in diabetes management
▪ Understanding CGM reports including the ambulatory glucose profile and time in range
d. Downloading Personal CGM and Sharing Data
People with diabetes who utilize a personal CGM can download their reports at home or view on a compatible smart device. The reports can be shared with their health care team by linking their individual account to the clinic’s account.
For those using a smart phone app, Bluetooth connectivity allows nearly constant download of real-time data. The data can also be downloaded during in-person clinic visits. See Compare CGMs for more information on downloading personal CGM device
data.
e. Interoperability
The FDA has an approval pathway that allows interoperability or use with other devices such as insulin pumps, closed loop systems, smart pens and mobile apps to qualify for expedited approval. Integrated CGM devices may have a less cumbersome approval process if they meet certain criteria.
f. Understanding CGM Data and Trends
It is important for individuals to focus not only on glucose in real time but also the direction and speed of the glucose trending. This includes not only proactively preventing hypoglycemia when blood glucose
is still in target ranges, but also when the direction and speed of the SG indicate a downward trend. Upward trending arrows and significant hyperglycemia can also be noted with potential for insulin pump or insertion site malfunction,
insufficient insulin, omission of a meal bolus or the need to change the timing of pre-meal insulin. Diabetes care and education specialists have an important role in discussing these concepts with the PWD so that the individual can
improve their time in range and reduce time spent in hypoglycemia and hyperglycemia in a shared decision-making relationship.
Glycemic variability should be assessed when downloading CGM data and should be included in patient discussions and recommendations for improvement.27 There is an international consensus statement that provides guidelines for CGM metrics. This includes a target range of 70-180mg/dL for all non-pregnant people with diabetes. The goal for most adults with Type 1 or Type 2 diabetes is to
spend over 70% time in range. The goal is to minimize time spent in hypoglycemia with no more than 4% of the time with glucose <70mg/dL.28
There is a CPT code for training PWD on personal CGM devices (CPT code 95249). CPT code 95250 is still the appropriate code for diagnostic or professional CGM. Although diabetes care and education specialists and/or RDs can perform services
associated with CPT codes 95249 or 95250, the billing must be done under a physician, nurse practitioner or PA. Medicare and most commercial payers limit RDs billing under their own NPI to diabetes self-management training (DSMT) and/or
medical nutrition therapy (MNT) services.
g. Use of CGM in Acute Care
The use of CGM in the hospital setting is not an FDA approved indication, although the FDA has allowed CGM use in hospitals during COVID-19.29 This allows for remote monitoring of patients to track episodes of hypoglycemia and hyperglycemia
and can save personal protective equipment (PPE) and time in sanitizing glucose meters. The benefit of a CGM device that alerts nurses that a patient’s glucose is rapidly rising or falling or that the insulin infusion rate needs
to be titrated would be of tremendous value. In the meantime, individuals may come to the hospital wearing their personal CGM device. The device should not be used for management decisions such as meal-time insulin dosing or hypoglycemia treatment in the hospital setting. In addition, only the hospital BG meter should be charted in the electronic health record.
CGMs that require calibration could be calibrated to the hospital meter so that the results are similar. Patients should be instructed to ask the nurse to verify the current SG with the hospital meter for meal time and correction insulin
dosing and treatment of hypoglycemia. In March, 2022, FDA granted Breakthrough Device Designation for Dexcom hospital CGM system. This designation allows for evaluation of CGM performance for the in-patient and provides a more efficient and streamlined review pathway for Dexcom CGM technology to be used in the hospital setting.30
h. Use of CGM in Special Populations
As CGM technology becomes more common in the pediatric population, personnel within the schools, camps and daycares will benefit from education on the technology to obtain a better understanding of their role in keeping children safe. School
diabetes medical management plans and 504 plans will need to be adapted to include the role of the CGM in the school environment and the responsibility of the school staff. Day and residential camps will need to determine how to utilize
the sensor information, as well as how to care for the receivers and keep them safe.
The issues of technology at camp, school and daycare are being reviewed and discussed nationwide,
and you are referred to Common Issues Involving Diabetes Care Tasks at School, Childcare, or Camp and Summer Camps to follow the recommendations in regards to incorporating sensors and other technology used to manage diabetes into these environments.
i. Sensor-Enhanced Pumps
Sensors are increasingly being linked to insulin pumps. Data from the sensor can be seen on the pump screen, and some pumps will respond to sensor data through automated insulin adjustments to basal and correction doses. It is vital that
the diabetes care and education specialist working with insulin pumps and sensors keep up to date on the options available to individuals and the functionality of the systems to increase the person’s understanding of the tools
to enhance their diabetes self-management skills. Here are some danatech resources that you can use for training and education to keep up with what is new.
To view available professional and personal CGMs, visit our Find & Compare CGMs page.
Using the ICC Framework and DATAA Tools to Optimize Care
The diabetes care and education specialist can use the Identify, Configure, Collaborate (ICC) framework to optimize care for people with diabetes.31 This includes identifying people with diabetes who would benefit from professional or
personal CGM, and then helping them to configure the device. This step can include setting appropriate high and low alerts, troubleshooting site adhesives and skin sensitivities, and coming up with a plan for how to respond to the
CGM numbers and arrows.
The collaborate step is where diabetes care and education specialists and PWD have data driven conversations about the data and through shared decision-making and come up with a plan to help increase time in
target range. There is a tool called DATAA which can help the diabetes care and education specialist systematically go through the data. This stands for (download the data, assess safety or hypoglycemia, time in range-focus on days/times
where time in range is highest and replicate the positive, areas for improvement, and action plan).
Conclusion
Diabetes care and education specialists who are well versed in professional and personal CGM devices are in a key position to promote and support this option for people with Type 1 and Type 2 diabetes. They can help identify those who
would benefit from these devices, collaborate with their provider to facilitate obtaining an appropriate device, educate them on the utilization, and download the results to evaluate in order to maximize diabetes management outcomes.
Diabetes care and education specialists provide the coaching needed to help PWD reap the benefits of the technology by utilizing the information to make smarter diabetes management decisions, reduce risk of hypoglycemia and hyperglycemia
and thereby improve quality of life.


 Guidance on clinical assessment, adjustments, report interpretation and key education. Designed to be used during clinic visits.
Guidance on clinical assessment, adjustments, report interpretation and key education. Designed to be used during clinic visits.-(3).jpg?sfvrsn=16486d59_5)
 Proper basal insulin initiation and titration can help reduce therapeutic inertia and engage patients in their diabetes management. Find videos, podcasts and other resources to fine tune your knowledge.
Proper basal insulin initiation and titration can help reduce therapeutic inertia and engage patients in their diabetes management. Find videos, podcasts and other resources to fine tune your knowledge.
 An overview of Glycemic Management Platforms that answers key questions including: What are they? What to consider? How can my inpatient facility benefit and more.
An overview of Glycemic Management Platforms that answers key questions including: What are they? What to consider? How can my inpatient facility benefit and more.


 Certificate programs, webinars and courses related to cardiometabolic health and diabetes. Topics include weight loss, treatments, lifestyle enhancements, the latest technological advancements and more.
Certificate programs, webinars and courses related to cardiometabolic health and diabetes. Topics include weight loss, treatments, lifestyle enhancements, the latest technological advancements and more. Find danatech's interactive diabetes technology tools for point-of-care, insurance coverage and more.
Find danatech's interactive diabetes technology tools for point-of-care, insurance coverage and more.